Amino acids are the building blocks of proteins, which are nitrogen-containing compound with an acidic carboxyl group (-COOH), a basic amino group (-NH2), a hydrogen atom, and a unique side chain or ‘R’ group. All covalently bonded to a carbon atom known as the α-carbon.
Except for glycine, all amino acids have an asymmetric carbon, leading to two optically active forms: “D” and “L.” Only the “L” forms are found in proteins.
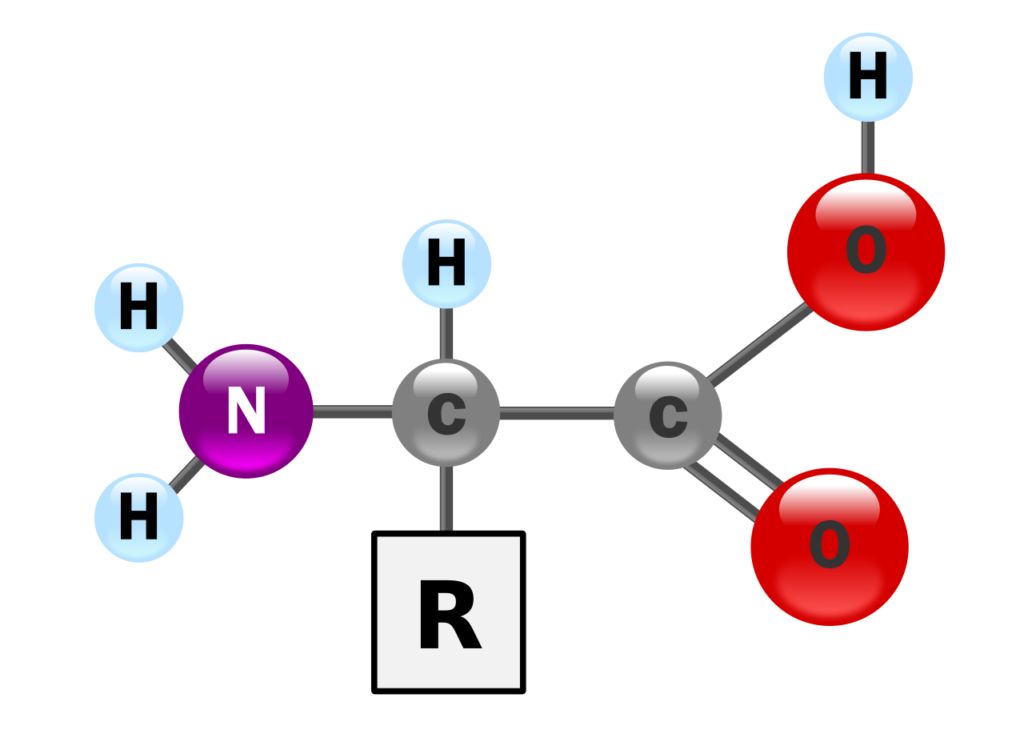
At neutral pH, amino acids exist as dipolar or zwitter ions. Proline and hydroxyproline, due to their cyclic structure with an imino group (NH), are classified as imino acids.
The side chains (R), which vary in size, shape, charge, hydrogen bonding, and chemical reactivity, give rise to 20 different amino acids found in proteins.
Structure of amino acids
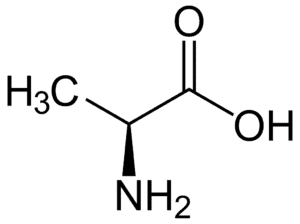
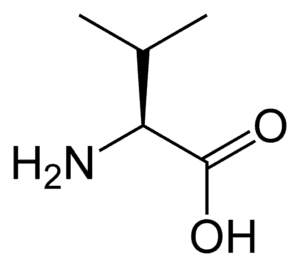
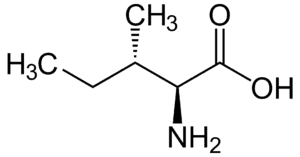
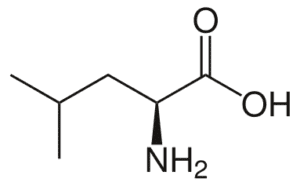
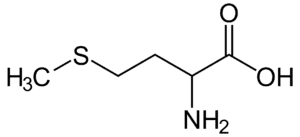
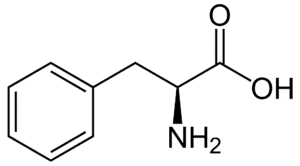
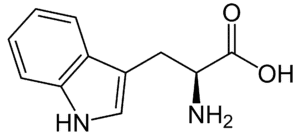
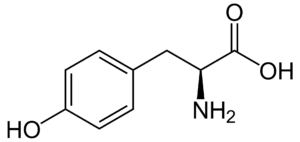
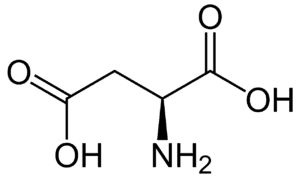
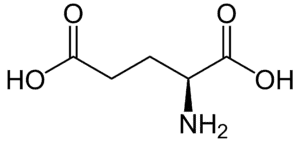
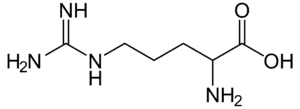
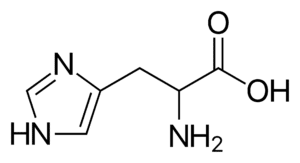
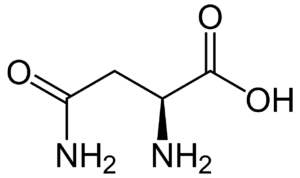
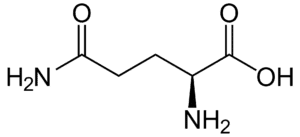
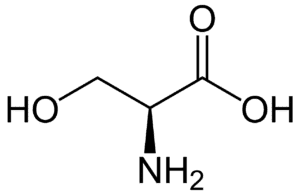
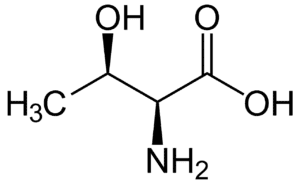
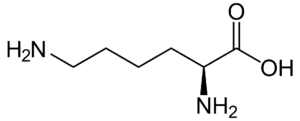
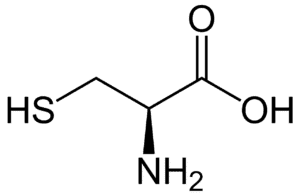
Fig: Structures of 20 different types of amino acids [Image Source: Wikipedia / Public Domain]
Properties of Amino Acids
Amino acids vary in their physical and chemical properties, which ultimately influence the characteristics of proteins.
Physical Properties:
- Solubility: Most of them are water-soluble but insoluble in organic solvents.
- Melting points: They generally have high melting points, often above 200°C.
- Taste: They can have different tastes: some are sweet (e.g., glycine, alanine, valine), others are tasteless (e.g., leucine), and some are bitter (e.g., arginine, isoleucine). Monosodium glutamate (MSG) enhances flavor.
- Optical properties: All of them, except glycine, have optical isomers due to the presence of an asymmetric carbon atom. Some, like isoleucine and threonine, have a second asymmetric carbon.
- Amino acids as ampholytes: Due to the presence of both acidic (-COOH) and basic (-NH2) groups, amino acids can act as ampholytes, donating or accepting protons.
Chemical Properties:
The chemical reactions of amino acids primarily result from the carboxyl (-COOH) and amino (-NH2) functional groups:
- Formation of salts and esters: Amino acids form salts (-COONa) with bases and esters (-COOR’) with alcohols.
- Reaction with ammonia: The carboxyl group of dicarboxylic amino acids reacts with NH3 to form amides.
- Amino group behavior: Amino groups act as bases and combine with acids (e.g., HCl) to form salts (-NH3+Cl–).
- Ninhydrin reaction: Alpha amino acids react with ninhydrin, producing a purple, blue, or pink complex known as Ruhemann’s purple.
- Oxidative deamination: They undergo oxidative deamination, releasing free ammonia.
- Reaction with acid chloride/anhydride: In an alkaline medium, they react with acid chloride or acid anhydride, producing “phthaloyl amino acids.”
- Edman degradation: When they react with Edman’s reagent, they form “phenyl thiohydantoin” and then cyclize into “phenyl thiohydantoin,” an Edman’s derivative.
Classification of amino acids
There are different ways of classifying the amino acids based on the structure and chemical nature, nutritional requirement, metabolic fate etc.
Classification of amino acids based on structure:
Amino acids can be classified comprehensively based on their structure and chemical properties. Each amino acid is represented by a three-letter or one-letter symbol commonly used in protein structure representations. The 20 amino acids found in proteins are divided into seven main groups:
- Amino acids with aliphatic side chains: These include mono-amino monocarboxylic acids. The group contains glycine, alanine, valine, leucine, and isoleucine. The last three (leucine, isoleucine, valine) have branched aliphatic side chains and are called branched-chain amino acids.
- Hydroxyl group-containing amino acids: Serine, threonine, and tyrosine belong to this group. Although tyrosine contains a hydroxyl group, it is often categorized with aromatic amino acids due to its aromatic nature.
- Sulfur-containing amino acids: Cysteine, with a sulfhydryl group, and methionine, with a thioether group, are the two sulfur-containing amino acids incorporated into proteins. Cystine, another sulfur-containing amino acid, forms by the condensation of two cysteine molecules.
- Acidic amino acids and their amides: Aspartic acid and glutamic acid are dicarboxylic mono-amino acids, while asparagine and glutamine are their amide derivatives. All four have distinct codons for protein incorporation.
- Basic amino acids: Lysine, arginine (with a guanidino group), and histidine (with an imidazole ring) are basic in nature, being dibasic monocarboxylic acids.
- Aromatic amino acids: Phenylalanine, tyrosine, and tryptophan (which contains an indole ring) are classified as aromatic amino acids. Histidine can also be included in this group.
- Imino acids: Proline, which contains a pyrrolidine ring, is unique among amino acids. It has an imino group (=NH) instead of the amino group (-NH2) found in other amino acids, making it an α-imino acid.
Classification of amino acids based on polarity:
They are categorized into four groups based on their polarity, which reflects their functional roles in protein structure.
- Non-polar amino acids: Also known as hydrophobic (water-repelling), they have no charge on their ‘R’ group. This group includes alanine, leucine, isoleucine, valine, methionine, phenylalanine, tryptophan, and proline.
- Polar amino acids with no charge on ‘R’ group: Although they carry no charge on their ‘R’ group, they contain functional groups like hydroxyl, sulfhydryl, or amide that participate in hydrogen bonding within proteins. Glycine, with its simple hydrogen ‘R’ group, is also part of this category. Glycine, serine, threonine, cysteine, glutamine, asparagine, and tyrosine lie in this group
- Polar amino acids with a positive ‘R’ group: This group includes lysine, arginine, and histidine, all of which have a positively charged ‘R’ group.
- Polar amino acids with a negative ‘R’ group: Aspartic acid and glutamic acid, which are dicarboxylic mono amino acids, belong to this group due to their negatively charged ‘R’ groups.
Classification of amino acids based on nutritional requirements:
The 20 amino acids are necessary for the production of various proteins and other biological functions. However, not all of them need to be obtained from the diet. Based on nutritional needs, they are classified into two categories: essential and non-essential.
- Essential or indispensable amino acids: These amino acids cannot be synthesized by the body and must be obtained from the diet. They are crucial for proper growth and maintenance. The ten essential amino acids are: arginine, valine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and tryptophan. Arginine and histidine can be synthesized by adults but not by growing children, so they are considered semi-essential. Thus, there are 8 absolutely essential amino acids and 2 semi-essential ones.
- Non-essential or dispensable amino acids: The body can produce around 10 amino acids to meet its biological needs, so they do not need to be consumed in the diet. These include glycine, alanine, serine, cysteine, aspartate, asparagine, glutamate, glutamine, tyrosine, and proline.
Classification of amino acids based on their metabolic fate:
The carbon skeleton of amino acids can be used as a precursor for the synthesis of glucose (glycogenic), fat (ketogenic), or both. From a metabolic perspective, they are classified into three groups:
- Glycogenic amino acids: These amino acids can act as precursors for the production of glucose or glycogen. Examples include alanine, aspartate, glycine, and methionine.
- Ketogenic amino acids: These amino acids contribute to fat synthesis. Leucine and lysine are ketogenic forms.
- Glycogenic and ketogenic amino acids: Amino acids such as isoleucine, phenylalanine, tryptophan, and tyrosine can serve as precursors for both glucose and fat synthesis.
Significance of amino acids
- The 20 essential amino acids are vital for life as they form peptides and proteins, serving as the building blocks of all living organisms.
- The linear arrangement of amino acid residues in a polypeptide chain dictates a protein’s three-dimensional structure, which, in turn, determines its function.
- They play a crucial role in maintaining human health by supporting hormone production, muscle structure, nervous system function, and the health of vital organs.
- Tissues utilize them to synthesize proteins and produce nitrogen-containing compounds (e.g., purines, heme, creatine, epinephrine), or they are oxidized for energy.
- The breakdown of dietary and tissue proteins produces nitrogen-containing substances and carbon skeletons.
- Nitrogen-containing compounds are used in the biosynthesis of purines, pyrimidines, neurotransmitters, hormones, porphyrins, and non-essential types.
- Carbon skeletons serve as fuel in the citric acid cycle, contribute to gluconeogenesis, or are used in fatty acid synthesis.