Lipids are organic compounds primarily made up of alcohol and fatty acids, bonded through ester linkages, usually insoluble in water but soluble in fats or organic solvents.
The plant they are present in seeds, nuts and fruits, while in animals they are stored in adipose tissues, bone marrow and nervous tissues. Chemically they are various types of esters of fatty acids and alcohol.
Addition to fatty acids and alcohols, some of the them may contain phosphoric acid, nitrogenous group and carbohydrate.
Lipids encompass substances such as fats, oils, waxes, and related compounds, and are found abundantly in both plants and animals.
Classification of Lipids
In 1943, Bloor proposed the following classification of lipids based on their chemical composition.
- Simple lipids
- Compound lipids
- Derived lipids
Simple Lipids
These are esters of fatty acid with various alcohols.
Fats and Oils
- They are referred to as neutral because they lack ionizable groups and therefore carry no charge.
- Neutral fats are the most common lipids in nature, making up about 98% of the lipids in adipose tissue, 30% of plasma or liver lipids, and less than 10% of erythrocyte lipids.
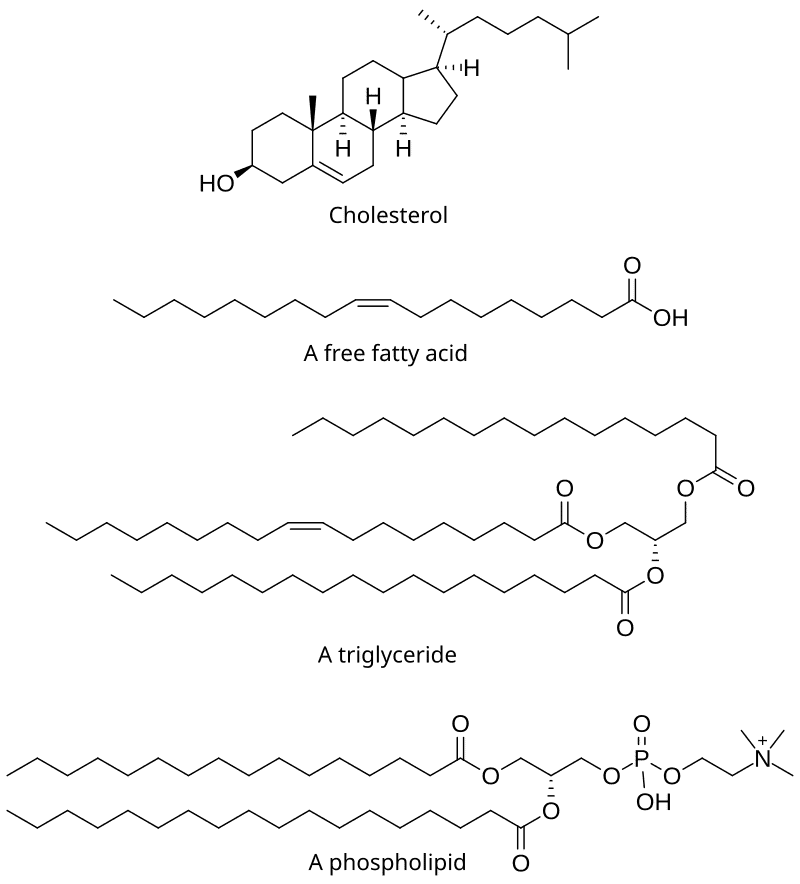
- These fats are esters formed from glycerol and various fatty acids. Since all three hydroxyl groups of glycerol are esterified, neutral fats are also known as “Triglycerides.”
- When glycerol is esterified with one fatty acid molecule, it forms a monoglyceride, and with two fatty acid molecules, it forms a diglyceride.
Types of triglycerides
- Simple triglycerides: When the three fatty acids attached to glycerol are identical, the triglyceride is referred to as a simple triglyceride, such as tripalmitin.
- Mixed triglycerides: When the fatty acids are different, the triglyceride is called a mixed triglyceride, examples include stearo-diolein and palmito-oleo-stearin.
Natural fats consist primarily of mixed triglycerides, with a small proportion of simple triglycerides. In animal fats, the most common fatty acids are palmitic, stearic, and oleic acids.
The key difference between fats and oils is that oils are liquid at room temperature, while fats are solid. This difference is largely due to oils having a higher percentage of unsaturated fatty acids, whereas fats contain mostly saturated fatty acids.
Physical properties of fat and oils
- Usually fats and oils are colorless, odorless, and tasteless. Any color or taste they have comes from several pigments.
- Fats have a specific gravity of less than 1, which allows them to float on water.
- Fats are insoluble in water but dissolve in organic solvents like ether and benzene.
- Fats generally have low melting points, though these are higher than their solidification points.
Chemical Properties of fats and oils
- Hydrolysis: Fats are broken down into their components (fatty acids and glycerol) through the action of superheated steam, acids, alkalis, or enzymes (e.g., lipase from the pancreas). Enzymatic and acid hydrolysis produce glycerol and free fatty acids.
- Saponification: Alkaline hydrolysis results in the formation of glycerol and fatty acid salts (soaps). Soaps help emulsify oily materials, making it easier to wash away fats.
- Halogenation: Neutral fats with unsaturated fatty acids can add halogens (such as iodine) at their double bonds. This property is used to measure the degree of unsaturation in fats or oils, which is important for determining their biological value.
- Hydrogenation or hardening of oils: This is an addition reaction where unsaturated fatty acids accept hydrogen at their double bonds. Hydrogenation occurs under high pressure with a catalyst, such as finely divided nickel or copper, and heat. This process is the basis for hardening oils, such as in margarine production, where liquid oleic acid is converted into solid stearic acid.
- Oxidation (Rancidity): This is a toxic reaction where triglycerides develop an unpleasant odor or taste due to oxidation by air, bacteria, or moisture. Rancidity is also the principle behind drying oils, like linseed oil, which is used in the production of paints and varnishes after exposure to atmospheric oxygen.
Waxes
Waxes are solid simple lipids consisting of a monohydric alcohol (with a molecular weight higher than glycerol) esterified to long-chain fatty acids. Examples of these alcohols include palmitoyl alcohol, cholesterol, and vitamins A or D.
Properties of waxes
- Waxes are insoluble in water but can dissolve in fat solvents, and they do not give a positive result in the acrolein test. (The acrolein test detects the presence of glycerol or fats.
- When fats are heated strongly with a dehydrating agent like potassium bisulphate (KHSO4), the glycerol part of the molecule is dehydrated to form acrolein, an unsaturated aldehyde with a sharp, irritating odor.)
- Waxes are not easily hydrolyzed as the fats and are indigestible by lipases and are very resistant to rancidity. Thus, they are of no nutritional value.
Type of Waxes
Waxes are found widely in nature, such as in the secretions of certain insects like beeswax, as protective coatings on the skin and fur of animals, and on the leaves and fruits of plants. They are categorized into true waxes and wax-like compounds:
- True waxes: Beeswax is secreted by honeybees and used to form honeycombs. It consists of a mixture of waxes, with mericyl palmitate as the main component.
- Wax-like compounds: Lanolin, or wool fat, is obtained from the skin glands associated with wool and is secreted by sebaceous glands. It is a complex mixture containing both free and esterified cholesterol, such as cholesterol-palmitate, along with other sterols.
Compound Lipids
These are lipids that include additional substances such as sulfur, phosphorus, amino groups, carbohydrates, or proteins, in addition to fatty acids and alcohol. Compound or conjugated lipids are categorized into the following types based on the nature of the additional group:
- Phospholipids
- Glycolipids
- Lipoproteins
Phospholipids
Phospholipids, also known as phosphatides, are compound lipids that include a phosphoric acid group in their structure. Their significance includes the following points:
- They are found in large quantities in the liver, brain, and blood, and every animal and plant cell contains phospholipids.
- The membranes surrounding cells and subcellular organelles are primarily composed of phospholipids, which control the transfer of substances through these membranes.
- They are crucial components of the lipoprotein coat necessary for the secretion and transport of plasma lipoprotein complexes, acting as lipotropic agents that help prevent fatty liver.
- The myelin sheath of nerves is rich in phospholipids.
- They play a vital role in the digestion and absorption of neutral lipids and in the excretion of cholesterol in bile.Phospholipids are important for blood clotting and platelet aggregation.
- They supply surfactants to lung alveoli, preventing their irreversible collapse.
- They are essential for signal transduction across the cell membrane.
They are present in all cells (both plant and animal) and can be found in milk and egg yolk as lecithins.
Classification of Phospholipids
They are classified into 2 groups according to the type of the alcohol present into two types: -Glycerophospholipids and Sphingophospholipids.
- Glycerophospholipids are the derivatives of phosphatidic acids which are simplest type of phospholipids and include: 1. Phosphatidic acids. 2. Lecithins 3. Cephalins. 4. Plasmalogens. 5. Inositides. 6. Cardiolipin.
- Sphingophospholipids are complex lipids formed when phosphate and fatty acids are linked to the alcohol sphingosine, with fatty acids connected via amide linkages rather than ester linkages. In seed lipids, phospholipids are present in smaller amounts compared to triacylglycerols, with phosphatidyl choline being the most abundant mammalian phospholipid. Sphingomyelins, the primary sphingophospholipids in animals, are absent in plants. Sphingomyelins are found in significant quantities in the brain and nerves, and in smaller amounts in the lungs, spleen, kidneys, liver, and blood. Unlike lecithins and cephalins, sphingomyelins contain sphingosine as the alcohol instead of glycerol. They include two nitrogenous bases: sphingosine itself and choline.
- Ceramide: This component of sphingomyelin is formed when the amino group of sphingosine is connected to the fatty acid through an amide linkage. Ceramides have been identified in their free state in the spleen, liver, and red blood cells.
Glycolipids
These are lipids that contain carbohydrate residues, with sphingosine serving as the alcohol and a very long-chain fatty acid (24 carbon series). They are found in cerebral tissue, which is why they are referred to as cerebrosides.
Classification of Glycolipids
Glycolipids are classified based on the number and type of carbohydrate residue(s) they contain, which includes cerebrosides, sulfatides, and gangliosides.
- Cerebrosides: These lipids are found in the myelin sheath of nerves, white matter of the brain, and cellular membranes. They are essential for nerve conduction.
- Sulfatides: These are sulfate esters of kerasin or phrenosin, with the sulfate group typically attached to the –OH group on C3 or C6 of galactose. Sulfatides are commonly found in the brain, liver, muscles, and testes.
- Gangliosides: These are more complex glycolipids located in the gray matter of the brain, ganglion cells, and red blood cells (RBCs). They facilitate the transfer of biogenic amines across cell membranes and function as cell membrane receptors.
Lipoproteins
Lipoproteins are lipids associated with proteins in tissues, with the lipid component consisting of phospholipids, cholesterol, or triglycerides. The bonds holding these components together are secondary bonds.
- Structural lipoproteins: These are found throughout various tissues, present in cellular and subcellular membranes. In lung tissue, they act as surfactants in a complex formed by protein and lecithin. In the eye, rhodopsin found in rod cells is a lipoprotein complex.
- Transport lipoproteins: These lipoproteins circulate in blood plasma and consist of a protein called apolipoprotein along with various types of lipids, including cholesterol, cholesterol esters, phospholipids, and triglycerides. As the lipid content increases, the density of plasma lipoproteins decreases.
Derived lipids
These substances are derived from simple and compound lipids through hydrolysis and include fatty acids, alcohols, mono- and diglycerides, steroids, terpenes, and carotenoids.
Cholesterol
- Cholesterol is the most significant sterol found in animal tissues, existing as either free alcohol or in an esterified form with fatty acids such as linoleic, oleic, or palmitic acids.
- It serves as the precursor for steroid hormones, bile salts, and vitamin D. Different tissues contain varying amounts of cholesterol, which plays both structural and metabolic roles; for instance, the adrenal cortex contains about 10%, the brain has around 2%, while other tissues contain 0.2-0.3%.
- Cholesterol is synthesized in the body from acetyl-CoA (approximately 1 g/day), and it is not found in plants.
- It is also obtained from the diet, providing around 0.3 g/day through sources like butter, milk, egg yolk, brain, meat, and animal fats.
Terpenes
- Terpenes are a large and varied class of organic compounds produced by many plants, especially conifers, as well as by certain insects such as termites and swallowtail butterflies, which emit terpenes from their osmeteria (defensive organs).
- Terpenes and terpenoids are key components of essential oils. These hydrocarbons and their oxygenated derivatives typically have fewer than 40 carbon atoms.
- The simplest terpenes are known as monoterpenes, with the formula C10H16; those with the formula C15H24 are called sesquiterpenes; C20H32 compounds are referred to as diterpenes, and C30H48 compounds are known as triterpenes.
- Terpenes with 40 carbon atoms (tetraterpenes) include compounds called carotenoids.
Biological Importance of Lipids
Lipids comprise a chemically varied group of compounds, and their biological functions are just as varied as their chemical structures.
- Lipids are more palatable and can be stored in unlimited amounts compared to carbohydrates. They have a high energy value, supplying about 25% of the body’s energy needs, and provide more energy per gram than carbohydrates and proteins, although carbohydrates are the preferred energy source.
- They supply essential fatty acids that the body cannot synthesize and provide fat-soluble vitamins (A, D, E, and K).
- They are vital components of the nervous system, with tissue fat being an essential part of cell membranes. This fat is primarily composed of phospholipids, which remain unaffected during starvation.
- Stored lipids, referred to as “depot fat,” are present in all human cells and serve several functions: they act as an energy reserve, cushion internal organs to protect them from external shocks, and provide subcutaneous thermal insulation to prevent heat loss.
- Lipoproteins, which are complexes of lipids and proteins, are crucial cellular components found in both cellular and subcellular membranes. Cholesterol is a key component of membrane structure and is utilized in the synthesis of adrenal cortical hormones, vitamin D3, and bile acids.
- It also play a role in addressing health issues such as obesity, atherosclerosis, lipid-storage diseases, essential fatty acid deficiency, and respiratory distress syndrome.
- Similarly, fats combined with proteins (lipoproteins) are important constituents of the cell membranes and mitochondria of the cell.
- They serve as structural components of the cell, creating a hydrophobic barrier that separates the aqueous contents of the cell and its subcellular structures.
- Despite being present in smaller amounts, they have essential functions as enzyme cofactors, electron carriers, light-absorbing pigments, and hydrophobic anchors for proteins.
- They also activate enzymes such as glucose-6-phosphatase, β-hydroxybutyric dehydrogenase, and stearyl CoA desaturase.
- It forms a protective waxy covering on the aerial parts of plants to check loss of water by evaporation.