Carbohydrates are polyhydroxy aldehydes or ketones, or compounds that produce these structures upon hydrolysis, with the general formula (CH₂O)n.
They are the most prevalent and one of the most important biomolecules made up of Carbon, Hydrogen and Oxygen in which Carbon is combined with Hydrogen and Oxygen in 1:2 ratio.
In most non-photosynthetic cells, the oxidation of carbohydrates serves as the primary pathway for energy production.
General Properties of Carbohydrates:
- Stereoisomerism: Compounds with identical structural formulas but different spatial arrangements. For instance, glucose has two stereoisomers concerning the penultimate carbon atom: D-glucose and L-glucose.
- Optical Activity: Refers to the ability of a substance to rotate plane-polarized light, resulting in (+) glucose and (−) glucose.
- Diastereoisomers: Variations in configuration at C2, C3, or C4 of glucose, such as mannose and galactose.
- Anomerism: The variation in spatial arrangement related to the first carbon atom in aldoses and the second carbon atom in ketoses.
- Osazone Formation: When sugars react with excess phenylhydrazine, they form osazones, which are derivatives of carbohydrates. For example, glucosazone is one such derivative.
- Oxidation: Monosaccharides can act as reducing sugars if their carbonyl groups are oxidized to carboxylic acids. In Benedict’s test, D-glucose is oxidized to D-gluconic acid, demonstrating that glucose is a reducing sugar.
- Reduction to Alcohols: The carbonyl groups in the open-chain forms of carbohydrates can be reduced to alcohols using sodium borohydride (NaBH4) or through catalytic hydrogenation. The resulting compounds are referred to as “alditols.”
Types of Carbohydrates
Carbohydrates can broadly be divided into three types viz, Monosaccharides, Oligosaccharides and Polysaccharides.
A. Monosaccharides
- The term “Monosaccharides” originates from the Greek words “Mono,” meaning single, and “saccharide,” meaning sugar.
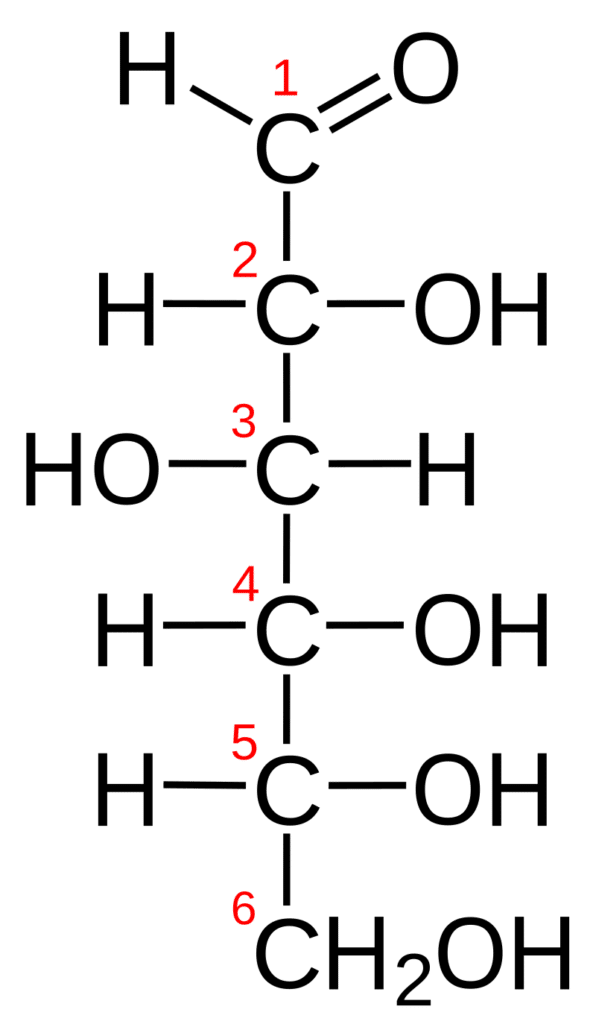
- Monosaccharides, also known as simple sugars, are composed of a single polyhydroxy aldehyde or ketone unit. The most common monosaccharide in nature is the six-carbon sugar D-glucose, often called dextrose.
- Monosaccharides are polyhydroxy aldehydes or ketones that cannot be hydrolyzed into simpler sugars.
- They are sweet-tasting, water-soluble, and crystalline in form.
- Monosaccharides contain 3 to 10 carbon atoms, 2 or more hydroxyl (OH) groups, and either an aldehyde (CHO) or a ketone (CO) group.
Classification of Monosaccharides
Monosaccharides are categorized in two ways: (a) based on the number of carbon atoms they contain, and (b) based on the type of carbonyl group they possess.
- Naturally occurring monosaccharides have between three to ten carbon atoms per molecule.
- The size of a monosaccharide can be identified by a name that combines a prefix indicating the number of carbon atoms with the suffix -ose.
- For example, triose, tetrose, pentose, and hexose refer to monosaccharides with three, four, five, and six carbon atoms, respectively.
- Monosaccharides are further classified as aldoses or ketoses.
- Aldoses: Those with an aldehyde functional group are called aldoses.
- Ketoses: While those with a ketone functional group on the second carbon atom are termed ketoses.
These classification methods of carbohydrates can be combined to form general names that reflect both the type of carbonyl group and the number of carbon atoms in the molecule.
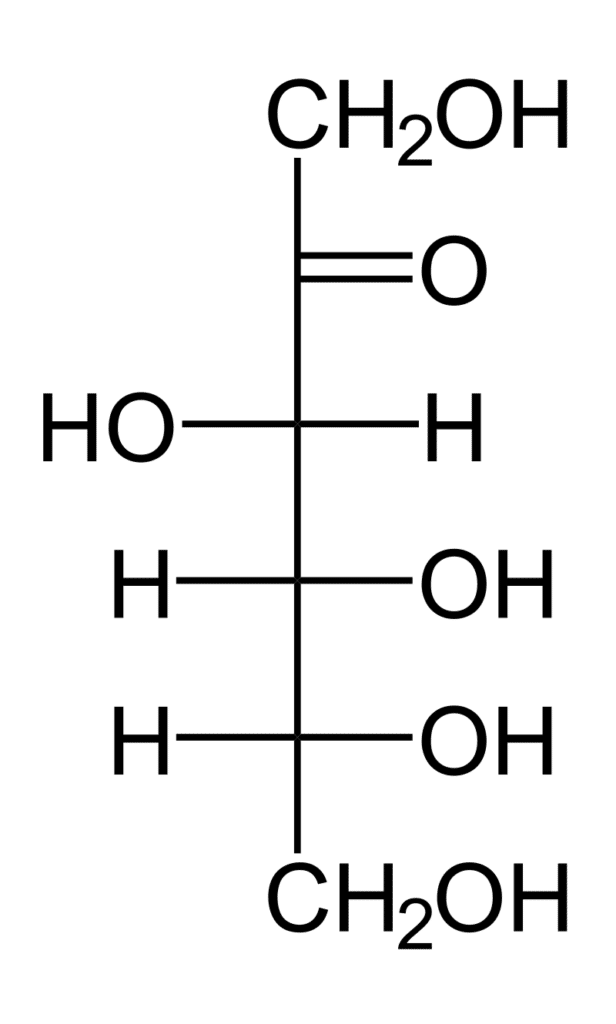
For instance, monosaccharides can be referred to as aldotetroses, aldopentoses, ketopentoses, ketoheptoses, etc. Glucose and fructose are examples of an aldohexose and a ketohexose, respectively.
Trioses
- Trioses are monosaccharides with 3 carbon atoms, and their molecular formula is C₃H₆O₃. Trioses are simple sugars. They are water-soluble. They have a sweet taste. A triose can have an aldehyde group (aldotriose) or a ketone group (ketotriose). Examples include glyceraldehyde and dihydroxyacetone.
Tetroses
- Tetroses are monosaccharides with 4 carbon atoms, and their molecular formula is C₄H₈O₄. Tetroses are simple sugars. They dissolve in water. They are sweet in taste. They are crystalline in nature. A tetrose can contain an aldehyde group (aldotetrose) or a ketone group (ketotetrose).
Pentoses
- Pentoses are monosaccharides with 5 carbon atoms, and their molecular formula is C₅H₁₀O₅. They are important components of nucleic acids. Pentoses are simple sugars. They are water-soluble. They taste sweet. They are crystalline in form. Pentoses can have an aldehyde group (aldopentose) or a ketone group (ketopentose).
Hexoses
- Hexoses are monosaccharides with 6 carbon atoms, and their molecular formula is C₆H₁₂O₆. Hexoses are simple sugars. They dissolve in water. They have a sweet taste. They are crystalline. A hexose can contain an aldehyde group (aldohexose) or a ketone group (ketohexose).
B. Oligosaccharides
- Oligosaccharides are composed of short chains of monosaccharide units, or residues, linked together by glycosidic bonds.
- The most common oligosaccharides are disaccharides, which consist of two monosaccharide units.
- An example is sucrose (cane sugar).
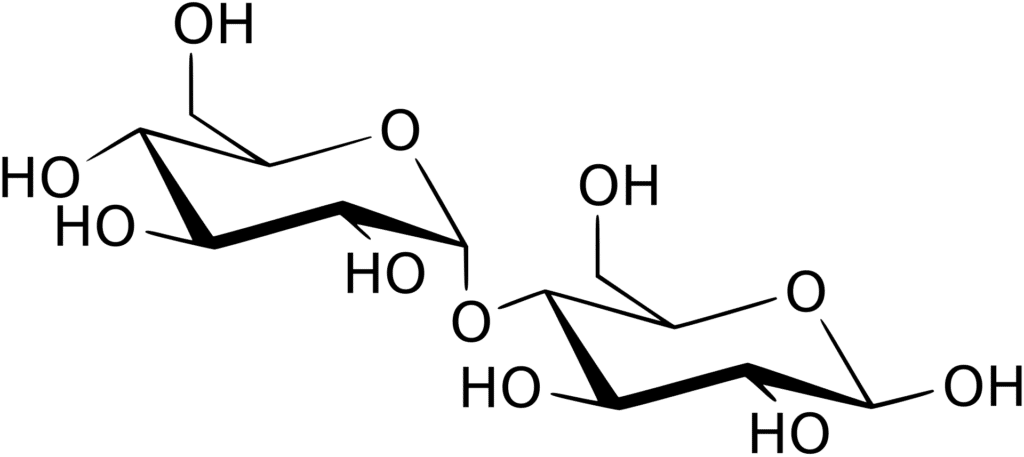
Disaccharides
- Disaccharides are made up of two sugar molecules connected by an O-glycosidic bond.
- The most prevalent disaccharides are sucrose, lactose, and maltose, while others include isomaltose, cellobiose, and trehalose.
- Disaccharides can be classified into two types, i.e Homodisaccharides and Heterodisaccharides.
C. Polysaccharides
- Polysaccharides are sugar polymers composed of more than 20 monosaccharide units, with some containing hundreds or even thousands of units.
- They are not reducing sugars because their anomeric carbons are linked through glycosidic bonds.
- Polysaccharides are categorized based on their function and composition. Functionally, they are classified into storage and structural types.
- Homopolysaccharides: Composed of only one type of monosaccharide unit.
- Heteropolysaccharides: Composed of more than one type of monosaccharide unit.
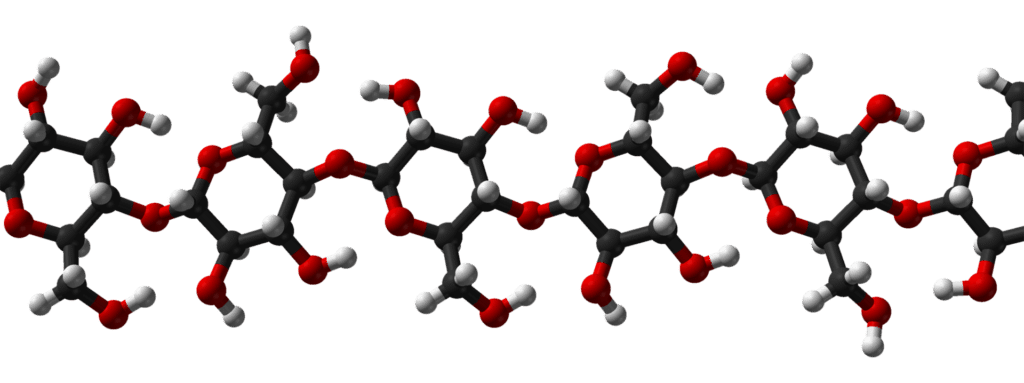
Starch
- Starch is a polymer made up of D-glucose units.
- Due to its high molecular weight, starch (and other glucose polymers) is usually insoluble in water but can form thick colloidal suspensions with water.
- Starch is a storage compound in plants, consisting of glucose units.
- It is a homopolysaccharide made up of two components: amylose and amylopectin.
- Most starch is composed of 10-30% amylose and 70-90% amylopectin.
- A straight-chain structure formed by 1,4 glycosidic bonds between α-D-glucose molecules.
Glycogen
- Glycogen is the storage polysaccharide in animals.
- It makes up to 10% of liver mass and 1-2% of muscle mass.
- Glycogen serves as stored energy for the organism.
- It is similar in structure to amylopectin, with the main difference being the number of branches.
- Glycogen has alpha(1,6) branches every 8-12 residues.
- Like amylopectin, glycogen produces a red-violet color when treated with iodine.
Cellulose
- β-glucose molecules are connected through condensation, which involves the removal of water, creating β-(1,4) glycosidic bonds.
- These glucose units form straight chains that range from 100 to 1,000 units long.
- Weak hydrogen bonds form between parallel chains, holding them together in cellulose microfibrils.
- These microfibrils bundle into thicker structures known as fibers.
- Cellulose fibers are often bonded by other substances like hemicelluloses and calcium pectate, contributing to the formation of plant cell walls.
- Due to the β-linkages, cellulose forms extended straight chains that hydrogen bond with each other, resulting in a highly rigid structure. Cellulose is a major structural polysaccharide and the most abundant organic compound on Earth, providing strength and rigidity to plant cell walls; wood consists of about 50% cellulose.
- Most animals lack the enzymes to break down cellulose, but it serves as dietary fiber that helps stimulate intestinal contractions and aids in moving food through the digestive system.
- Some animals, such as cows, sheep, and horses, can digest cellulose with the help of bacteria in their digestive systems that convert cellulose into glucose. Ruminants use multiple stomachs to allow more time for digestion, while animals like rabbits reprocess their food to facilitate further cellulose breakdown.
- Cellulose is crucial in various industries, being a key component in products like wood, paper, cotton, cellophane, rayon, linen, nitrocellulose (gun cotton), and photographic films (cellulose acetate).
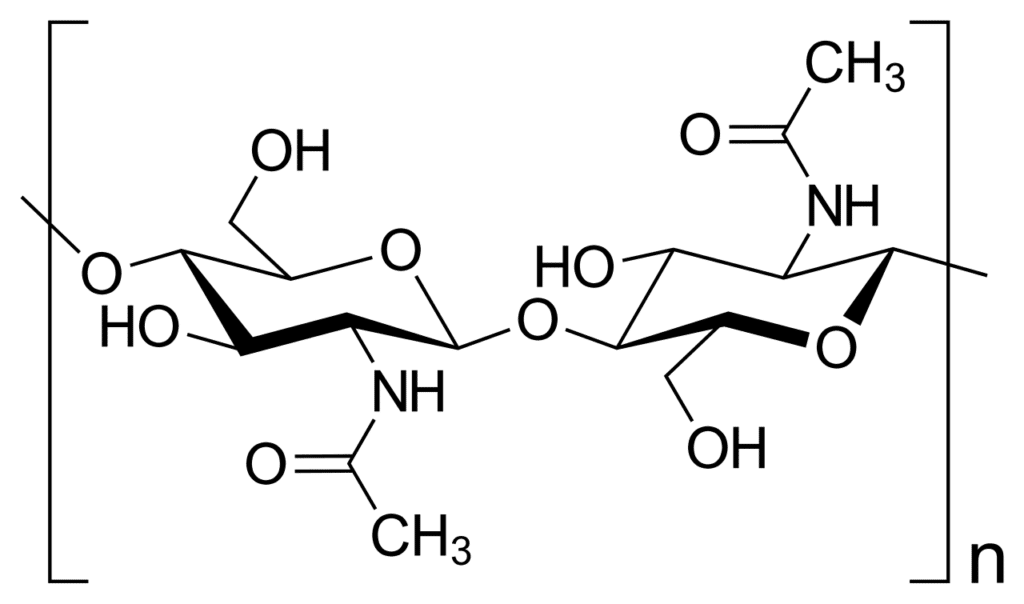
Chitin
- Chitin is a polymer found in diverse environments, including beetle shells and spider webs. It is present in both plants and animals.
- Chitin is sometimes considered a derivative of cellulose due to their molecular similarities.
- While cellulose contains a hydroxyl group, chitin contains an acetamide group.
- Chitin is a natural polymer, existing in nature rather than being man-made. It is found in high quantities in crabs, beetles, worms, and mushrooms.
- Chitin is a tough material that helps protect insects from damage and pressure.
Biological Significance of Carbohydrates
- Carbohydrates are a primary energy source, providing an immediate energy supply for many animals.
- Glucose is metabolized to produce ATP.
- Glucose serves as an energy storage molecule, stored as glycogen in animals and as starch in plants.
- Stored carbohydrates provide an alternative energy source, sparing proteins from being used for energy.
- Carbohydrates act as intermediates in the biosynthesis of fats and proteins.
- Carbohydrates play a role in regulating nerve tissue and serve as the main energy source for the brain.
- Carbohydrates combine with lipids and proteins to form surface antigens, receptor molecules, vitamins, and antibiotics.
- They contribute to structural and protective roles, such as in the cell walls of plants and microorganisms.
- In animals, carbohydrates are key components of connective tissues.
- They are involved in biological transport, cell-to-cell communication, and the activation of growth factors.
- Carbohydrates high in fiber content help prevent constipation.
- They also assist in modulating the immune system.