Nitrogen fixation is the process of transformation of atmospheric free nitrogen (N2) into nitrogenous components that can be used by biological entities. It is vital for ecological balance, enhancing soil fertility, nutrient cycling, and plant growth.
Nitrogen gas is highly stable because of the strong triple bond between its nitrogen atoms, and breaking this bond demands a significant amount of energy which at least sixteen ATP molecules. Only a select group of prokaryotes are able to carry out this energetically demanding process.
Most of the N2 fixation during nitrogen cycle, is carried out by prokaryotes, whereas some nitrogen can also be fixed physically by lightning or by certain industrial processes.
Types of Nitrogen Fixation
Nitrogen fixation can broadly be divided into two types;
- Non-Biological Nitrogen Fixation
- Biological Nnitrogen Fixation
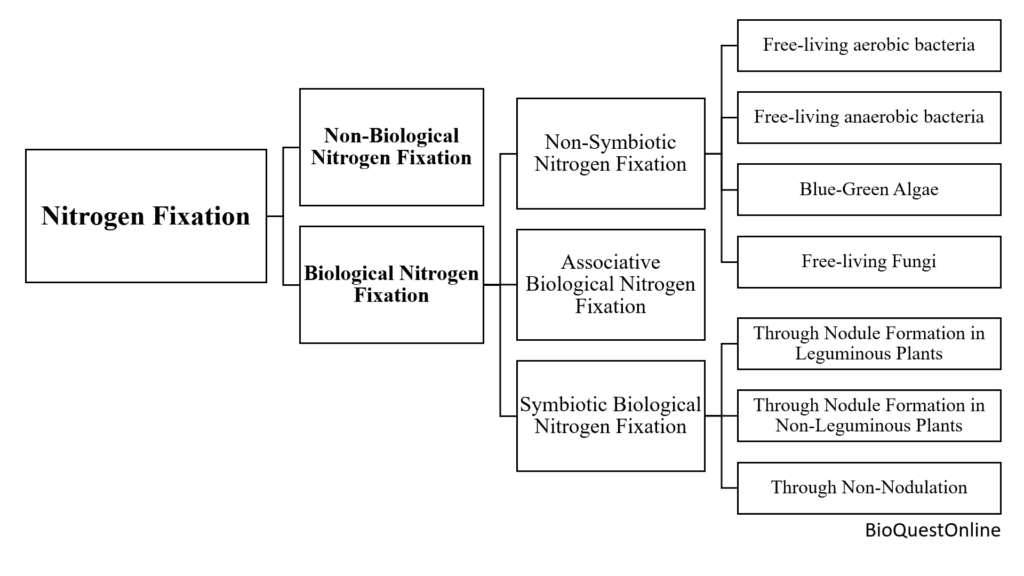
Non-Biological Nitrogen Fixation
Nitrogen can be fixed by lightning, which converts nitrogen and oxygen in the atmosphere into nitrogen oxides. These oxides may react with water or rain to form nitrous acid or nitric acid, which then seeps into the soil, creating nitrates that plants can utilize.
Nitrogen in the atmosphere is highly stable and unreactive due to the strong triple bond between atoms in the N2 molecule. However, lightning generates enough energy and heat to break this bond, allowing nitrogen atoms to react with oxygen.
Although the initial compounds formed cannot be used by plants, as these molecules cool, they react with oxygen to form NO2. This NO2 then reacts with water to produce HNO3 (nitric acid) or its ion NO3− (nitrate), which is usable by plants.
Biological Nitrogen Fixation
Biological nitrogen fixation was first discovered by German agronomist Hermann Hellriegel and Dutch microbiologist Martinus Beijerinck. This process occurs when atmospheric nitrogen is converted into ammonia by the enzyme nitrogenase.
It serves as a valuable alternative to nitrogen fertilizers. It is naturally carried out in soil by microorganisms known as diazotrophs, which include bacteria like Azotobacter and certain archaea.
All forms of biological nitrogen fixation are facilitated by nitrogenase enzymes, which contain iron and often another metal, usually molybdenum, though sometimes vanadium.
Biological nitrogen fixation can be categorized into three types:
- Non-Symbiotic/Asymbiotic B2 Nitrogen Fixation
- Associative Biological N2 Fixation
- Symbiotic Biological N2 Fixation.
Non- Symbiotic/ Asymbiotic Biological Nitrogen Fixation
Soil contains various free-living nitrogen-fixing organisms, including several aerobic and anaerobic bacteria, as well as blue-green algae.
Biological nitrogen fixation by microorganisms that live freely or outside of plant cells is known as non-symbiotic Biological N2 Fixation. The asymbiotic nitrogen fixers can be categorized as follows:
- Free-living aerobic nitrogen-fixing bacteria: Photosynthetic: Chlorobium, Chromatium and Non-Photosynthetic: Azotobacter, Azomonas, Derxia, Beijerinckia
- Free-living anaerobic nitrogen-fixing bacteria: Photosynthetic: Rhodospirillum and Non-Photosynthetic: Clostridium
- Blue-Green Algae: Nostoc, Anabaena, Rivularia, Calothrix, Oscillatoria, Gloeocapsa, Lyngbya, Plectonema etc.
- Free-living Fungi: Yeasts and Pullularia are free living fungi which perform non-symbiotic nitrogen fixation
These asymbiotic, free-living nitrogen fixers are considered quite primitive. The fixation process is a reduction reaction that occurs independently of respiration. These organisms are more active in fixing nitrogen under low oxygen conditions, as long as no hydrogen gas is produced.
Associative Biological Nitrogen Fixation
- Certain bacteria that live in close contact with the roots of cereals and grasses are capable of fixing nitrogen. This relationship, known as associative symbiosis, is a form of loose mutualism.
- The bacteria inhabit the transition zone between the soil and the roots (the rhizosphere) and may occasionally enter the roots. Some of the fixed nitrogen is absorbed by the roots, while the bacteria benefit from the carbohydrates released by the roots.
- Examples of this association include: Azospirillum brasilense with cereal roots. Beijerinckia with the roots of sugarcane. Azotobacter paspali with the roots of the tropical grass Paspalum notatum.
Symbiotic Biological Nitrogen Fixation
Symbiotic biological N2 fixation occurs within a mutualistic relationship where plants provide a habitat and fixed carbon to bacteria in exchange for fixed nitrogen.
This ecological association between bacteria and their host is long-term and beneficial for both parties, with the microbial partner responsible for fixing atmospheric nitrogen.
Examples of symbiotic biological nitrogen fixation can be grouped into three categories:
Nitrogen Fixation through Nodule Formation in Leguminous Plants
- In many legume plants, symbiotic nitrogen fixers, mainly from the genus Rhizobium, establish themselves inside specialized root structures called root nodules.
- The bacteria only fix nitrogen when they are inside these nodules. This relationship is symbiotic because the host plant provides the nodule bacteria with organic carbon (carbohydrates), while the bacteria supply the plant with fixed nitrogen.
- For example, Bradyrhizobium japonicum is a slow-growing symbiont of soybeans, and Azorhizobium caulinodans forms stem nodules in Sesbania species.
Nitrogen Fixation through Nodule Formation in Non-Leguminous Plants
- Some plants outside the Leguminosae family also produce root nodules and fix nitrogen, particularly certain trees and shrubs.
- Key examples include:The genus Frankia, which forms root nodules in association with Alnus, Casuarina equisetifolia, and Myrica gale. Rhizobium forms root nodules in the genus Parasponia. Leaf nodules are formed by Klebsiella in the genus Psychotria and by Burkholderia in Pavetta zimermanniana.
Nitrogen Fixation through Non-Nodulation
- In some plants, during nitrogen cycle, symbiotic N2 fixation occurs without the formation of nodules, a situation known as pseudo-symbiosis.
- Examples include: Lichens, which involve a symbiosis between fungi and algae (cyanobacteria or green algae). Anthoceros, a bryophyte, in association with Nostoc. Azolla, a fern, in association with Anabaena. Cycas, a gymnosperm, in association with Anabaena or Nostoc in its coralloid roots. The roots of Digitaria, Sorghum, and Maize in association with Spirillum.
Importance of Nitrogen Fixation
- Nitrogen fixation plays a vital role in maintaining the ecological balance and supports ecosystem sustainability and productivity. It is fundamental to plant growth, impacts nutrient cycling, and enhances soil fertility.
- As a natural alternative to chemical fertilizers, which are costly and environmentally harmful, it relies on bacteria that either form symbiotic relationships with plants or exist freely in the soil. In legumes, these bacteria create nodules on the roots, improving the plant’s ability to access nitrogen.
- Nitrogen is an essential macronutrient for plant development, forming a key component of proteins, amino acids, and nucleic acids. However, atmospheric nitrogen primarily exists as inert gas, which plants cannot utilize directly.
- Leguminous plants, such as soybeans, peas, and clover, are particularly known for their root nodules that house nitrogen-fixing bacteria. These bacteria provide the plants with ammonia, a usable form of nitrogen, thereby fostering their growth and development.
- Non-leguminous plants also indirectly benefit from nitrogen fixation as the nitrogen fixed by bacteria becomes available to them through processes like mineralization and decomposition.
- Biological N2 supports biodiversity by enabling the growth of nitrogen-fixing plants and enhances the nutritional quality of plant tissues by increasing protein content, which is crucial for plant and animal nutrition.
- Leguminous crops are highly valued not only for their nitrogen-fixing abilities but also for their protein-rich composition.
- In aquatic ecosystems, nitrogen-fixing organisms contribute to nitrogen cycling, ensuring the availability of nitrogen for aquatic plants and other organisms in freshwater and marine environments.
You Might Be Interested In:
Nitrogen Cycle; Its 5 Stages and Importance
FREQUENTLY ASKED QUESTIONS (FAQS)
What is nitrogen fixation?
Nitrogen fixation is the process of transformation of atmospheric free nitrogen (N2) into nitrogenous components that can be used by biological entities. Most of the N2 fixation during nitrogen cycle, is carried out by prokaryotes, whereas some nitrogen can also be fixed physically by lightning or by certain industrial processes.
What are the types of nitrogen fixation?
Most of the N2 during nitrogen cycle, is carried out by prokaryotes, whereas some nitrogen can also be fixed physically by lightning or by certain industrial processes.
Nitrogen fixation can broadly be divided into two types;
1. Non-Biological N2 Fixation
2. Biological N2 Fixation
What is non biological nitrogen fixation?
Nitrogen can be fixed by lightning, which converts nitrogen and oxygen in the atmosphere into nitrogen oxides. These oxides may react with water or rain to form nitrous acid or nitric acid, which then seeps into the soil, creating nitrates that plants can utilize. Such process is called as non biological nitrogen fixation.
Although the initial compounds formed cannot be used by plants, as these molecules cool, they react with oxygen to form NO2. This NO2 then reacts with water to produce HNO3 (nitric acid) or its ion NO3− (nitrate), which is usable by plants.
Define biological nitrogen fixation.
Biological nitrogen fixation is the process in which atmospheric nitrogen is converted into ammonia by the enzyme nitrogenase. All forms of biological N2 fixation are facilitated by nitrogenase enzymes, which contain iron and often another metal, usually molybdenum, though sometimes vanadium.
Biological N2 fixation (BNF) serves as a valuable alternative to nitrogen fertilizers. It is naturally carried out in soil by microorganisms known as diazotrophs, which include bacteria like Azotobacter and certain archaea.
What are three types of biological nitrogen fixation?
Biological nitrogen fixation can be categorized into three types:
1. Non-Symbiotic/Asymbiotic Biological N2 Fixation
2. Associative Biological N2 Fixation
3. Symbiotic Biological N2 Fixation.
What is symbiotic biological nitrogen fixation?
This ecological association between bacteria and their host is long-term and beneficial for both parties, with the microbial partner responsible for fixing atmospheric nitrogen.
Symbiotic nitrogen fixation occurs within a mutualistic relationship where plants provide a habitat and fixed carbon to bacteria in exchange for fixed nitrogen.
How do symbiotic biological nitrogen fixation occur?
Symbiotic biological nitrogen fixation by following ways;
1. Through Nodule Formation in Leguminous Plants
2. Through Nodule Formation in Non-Leguminous Plants
3. Through Non-Nodulation
Why is nitrogen fixation important?
1. Nitrogen fixation is vital for ecological balance, enhancing soil fertility, nutrient cycling, and plant growth.
2. It serves as an eco-friendly alternative to chemical fertilizers by relying on nitrogen-fixing bacteria, which form root nodules in legumes like soybeans and clover, providing plants with usable nitrogen.
3. Non-leguminous plants benefit indirectly through processes like decomposition.
4. Nitrogen fixation supports biodiversity, boosts plant protein content, and enriches leguminous crops.
5. In aquatic ecosystems, nitrogen-fixing organisms aid nitrogen cycling, benefiting aquatic plants and sustaining freshwater and marine ecosystems.


