Nucleic acids are biopolymers made up of mononucleotides, which form their repeating units, composed of carbon, hydrogen, oxygen, nitrogen, and phosphorus, serve as the genetic material in living organisms.
Being long-chain polymeric molecules, their monomers are called nucleotides, which is why nucleic acids are sometimes referred to as polynucleotides. These macromolecules are found in nearly all living cells, either freely or attached to proteins as nucleoproteins.
Types of Nucleic Acids
Nucleic acids are of two types;
Deoxyribonucleic Acid (DNA)
- DNA consists of a pentose sugar, phosphoric acid, and nitrogen-containing cyclic bases.
- The sugar component in DNA is β-D-2-deoxyribose.
- The nitrogenous bases found in DNA are adenine (A), guanine (G), cytosine (C), and thymine (T).
- The arrangement of these bases within the DNA molecule is crucial for the transmission of genetic information from one generation to the next.
- DNA has a double-stranded helical structure, with the two strands being complementary to each other.
Ribonucleic Acid (RNA)
- RNA is also made up of phosphoric acid, a pentose sugar, and nitrogen-containing cyclic bases.
- The sugar present in RNA is β-D-ribose.
- The nitrogenous bases found in RNA include adenine (A), guanine (G), cytosine (C), and uracil (U), with uracil replacing thymine found in DNA.
- RNA typically exists as a single strand, though it can fold back on itself, forming regions with a double helix structure.

Functions of Nucleic Acids
- Nucleic acids play a key role in protein synthesis within the body.
- RNA is crucial for the process of protein production.
- Loss of DNA is associated with various diseases.
- DNA is essential for passing genetic information from parents to their offspring.
- All cellular information is stored in DNA.
- DNA fingerprinting is a technique used in forensics to determine paternity and to identify criminals.
Components of Nucleic acid
Upon hydrolysis, under different set of conditions nucleic acids yield 3 components: phosphoric acid, a pentose sugar and nitrogenous bases.
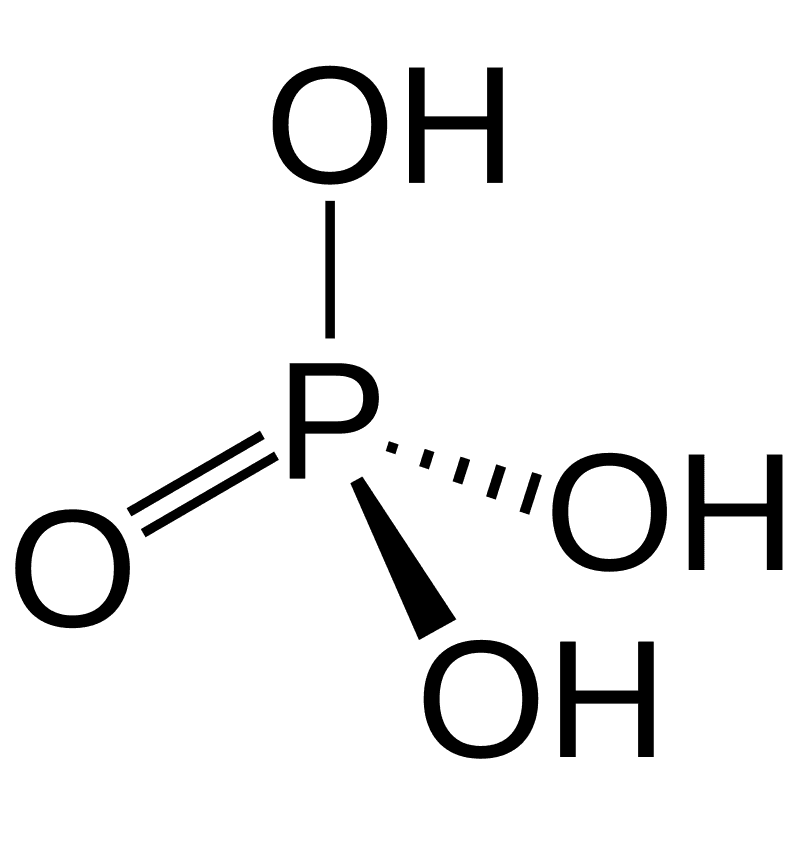
Phosphoric Acid
- The Phosphate group (PO43-) in the nucleotides is derived from Phosphoric acid.
- Phosphoric acid has the molecular formula H3PO4.
- It consists of three monovalent hydroxyl groups and one divalent oxygen atom, all bonded to a pentavalent phosphorus atom.
Nitrogenous Bases
- Nitrogenous bases belong to the heterocyclic group of organic compounds and have a planar structure.
- In all nucleic acids, two types of nitrogenous bases are present.
- These bases are attached to the sugar moiety at the same carbon atom (C1) involved in sugar-sugar bonds.
- The nitrogenous bases are derivatives of pyrimidine and purine, and due to their π electron clouds, both pyrimidine and purine bases form planar molecules.
Pyrimidine Derivatives
These compounds are all derived from the parent heterocyclic compound pyrimidine, which consists of a six-membered ring with two nitrogen atoms and three double bonds. Pyrimidine has a melting point of 22°C and a boiling point of 123.5°C. The common pyrimidine derivatives found in nucleic acids are uracil, thymine, and cytosine.
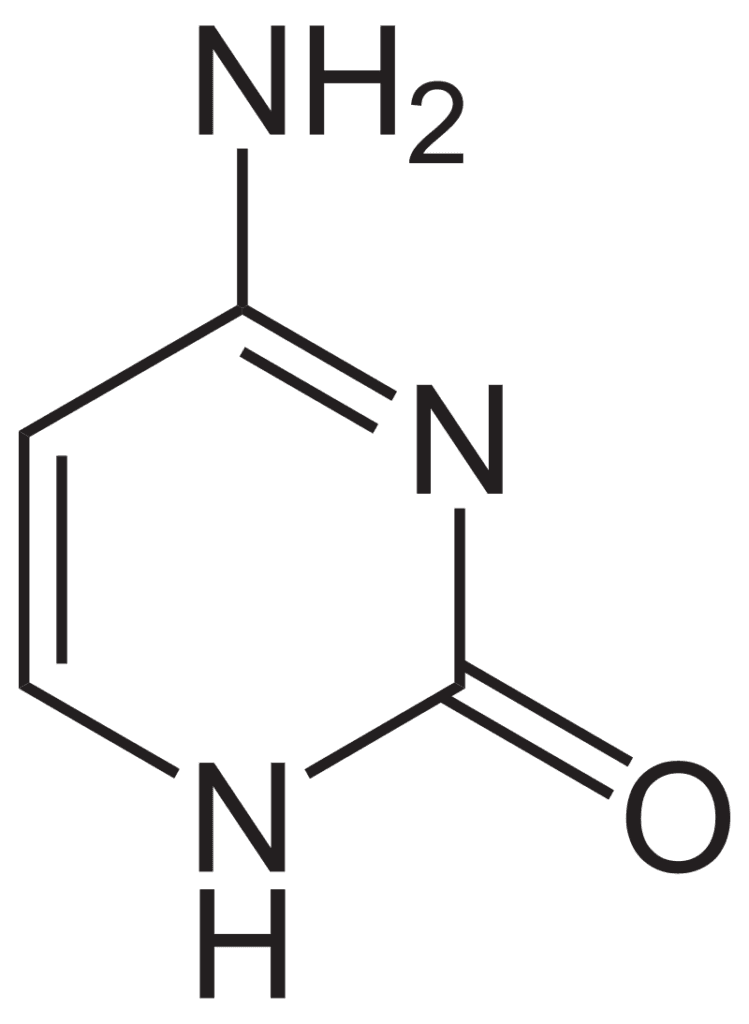

- Uracil: Uracil (C4H4O2N2), present only in RNA, is a white crystalline pyrimidine base with a molecular weight of 112.10 daltons and a melting point of 338°C. Uracil rarely occurs in DNA.
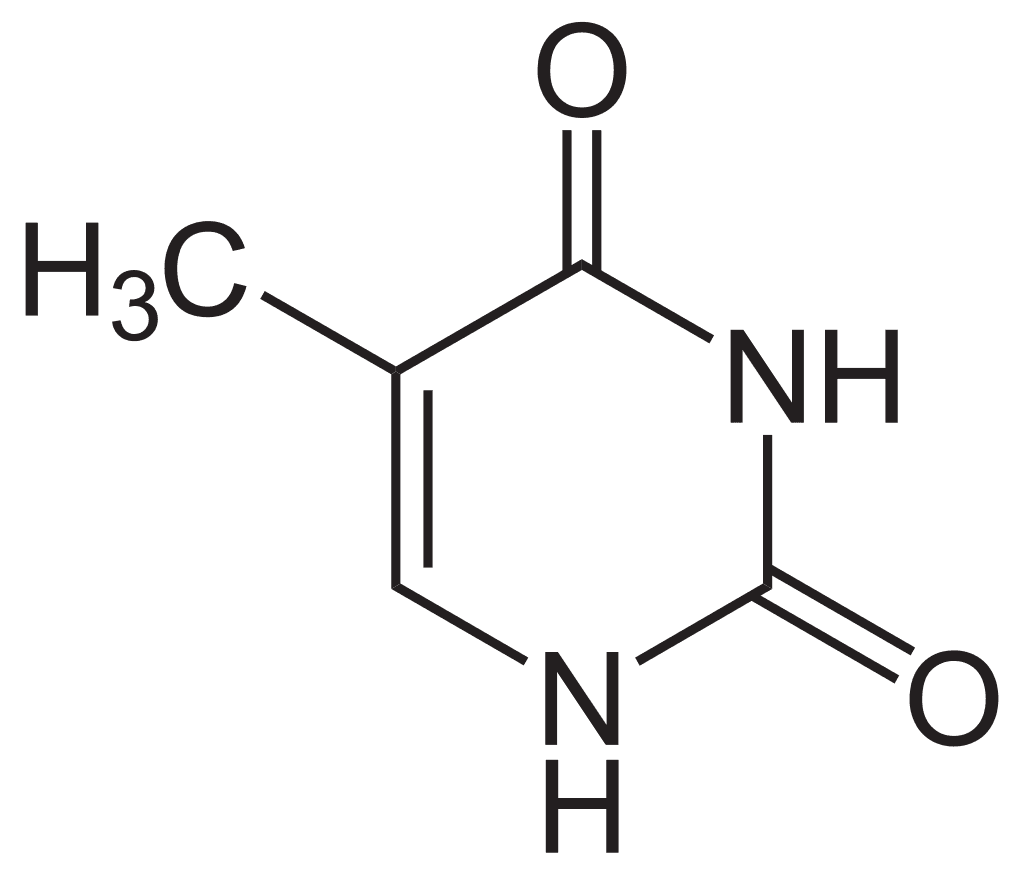
- Thymine: Thymine (C5H6O2N2), exclusive to DNA, has a molecular weight of 126.13 daltons and was first isolated from the thymus, hence its name. Thymine is rarely found in RNA.
- Cytosine: Cytosine (C4H5ON3) is present in both RNA and DNA. It is a white crystalline substance with a molecular weight of 111.12 daltons and a melting point between 320°C and 325°C.
Purine Derivatives
These compounds are derived from their parent compound, purine, which consists of a six-membered pyrimidine ring fused with a five-membered imidazole ring and is structurally related to uric acid. Purine has a melting point of 216°C. The common purine derivatives found in nucleic acids are adenine and guanine.
- Adenine: Adenine (C5H5N5), present in both RNA and DNA, is a white crystalline purine base with a molecular weight of 135.15 daltons and a melting point of 360-365°C.
- Guanine: Guanine (C5H5ON5), also present in both RNA and DNA, is a colorless, insoluble crystalline substance with a molecular weight of 151.15 daltons. It was originally isolated from guano, which is where its name comes from.


Sugar
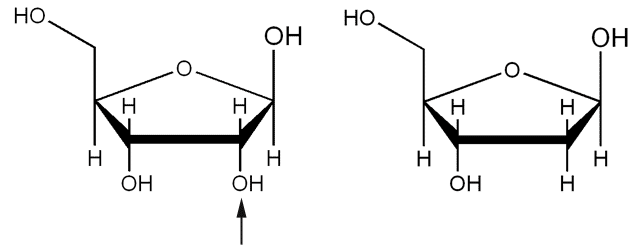
The two types of nucleic acids are mainly distinguished by the 5-carbon keto sugar, or pentose, they contain. One contains D-2-deoxyribose, giving it the name deoxyribonucleic acid (DNA), while the other contains D-ribose, hence the name ribonucleic acid (RNA). Both sugars in nucleic acids are in the furanose form and have a β configuration.
- Upon examining the structures of the two sugars, D-ribose is the parent sugar, while D-2-deoxyribose is a derivative in which the OH group on carbon 2 is replaced by a hydrogen atom.
- These sugars can be differentiated through specific color reactions: ribose reacts with orcinol in hydrochloric acid with ferric chloride, while deoxyribose reacts with diphenylamine in an acidic solution.
- A key property of pentoses is their ability to form esters with phosphoric acid. In this process, the OH groups on carbons 3 and 5 of the pentose participate, forming a 3′, 5′-phosphodiester bond between adjacent pentose units, which is a fundamental part of nucleic acid structure.
Nucleosides
- Nucleosides are compounds formed when nitrogenous bases (purines and pyrimidines) are linked to pentose sugars (ribose or deoxyribose) through a β-glycosidic bond.
- This configuration is also present in polymeric nucleic acids. The β-glycosidic bond involves the C-1′ carbon of the sugar and the hydrogen atom of N-9 (for purines) or N-1 (for pyrimidines), resulting in the elimination of a water molecule.
- Consequently, purine nucleosides are classified as N-9 glycosides, while pyrimidine nucleosides are N-1 glycosides. Similar to O-glycosides, nucleosides are stable in alkaline conditions.
- Purine nucleosides can be easily hydrolyzed by acids, whereas pyrimidine nucleosides require prolonged exposure to concentrated acid for hydrolysis.
- Nucleosides are typically named after the specific purine or pyrimidine they contain. Those with ribose are referred to as ribonucleosides, while those with deoxyribose are called deoxyribonucleosides.

Nucleotides
- Nucleotides are the phosphoric acid esters of nucleosides, occurring either in free form or as subunits within nucleic acids. As previously mentioned, the phosphate group is always esterified to the sugar component.
- In the ribose portion of a ribonucleoside, phosphorylation can occur at three positions (C2′, C3′, C5′), as C1′ and C4′ are part of the furanose ring formation. This means that the phosphate group can only be esterified at these three sites.
- Whereas, in the deoxyribose component of a 2′-deoxyribonucleoside, only two positions (C3′ and C5′) are available for phosphorylation because C1′ and C4′ are involved in the furanose ring, and C2′ lacks a hydroxyl group.
- Consequently, the hydrolysis of RNA and DNA through various methods and under different conditions yields isomeric nucleotides of three types for RNA and two types for DNA.
- While the names of nucleosides and nucleotides are typically derived from their corresponding bases, there is one exception: the base associated with the nucleoside called inosine (and its derived nucleotides) is known as hypoxanthine.
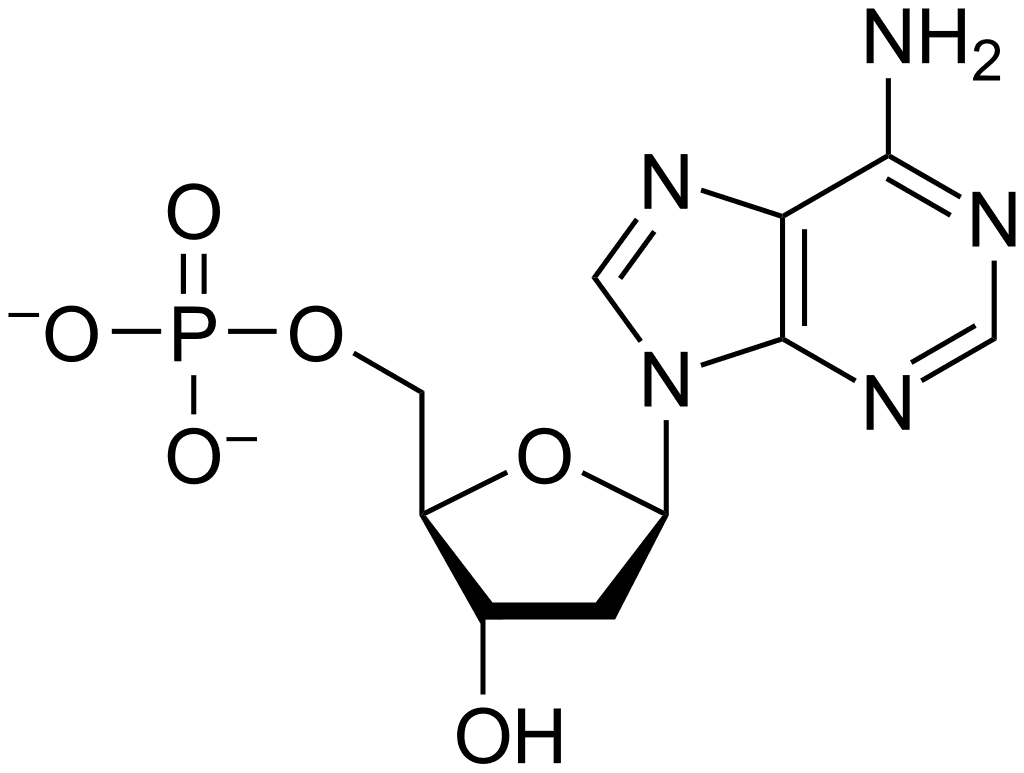
Functions of Nucleotides
Carriers of Chemical Energy:
- Nucleotides can have one, two, or three phosphate groups covalently attached to the 5′-OH of ribose. These are known as nucleoside mono-, di-, and triphosphates, abbreviated as NMPs, NDPs, and NTPs, respectively.
- The three phosphate groups are labeled as α, β, and γ, starting from the ribose. NTPs serve as a source of chemical energy for numerous biochemical reactions, with adenosine triphosphate (ATP) being the most widely utilized.
- Other nucleotides, such as uridine triphosphate (UTP), guanosine triphosphate (GTP), and cytidine triphosphate (CTP), are employed in specific reactions.
- The hydrolysis of ATP and other nucleoside triphosphates is an exergonic reaction. The bond between ribose and the α-phosphate is an ester linkage, while the α-β and β-γ linkages are phosphoric acid anhydrides.
- Hydrolyzing the ester linkage releases about 14 kJ/mol, while breaking each anhydride bond releases approximately 30 kJ/mol. In biosynthesis, ATP hydrolysis often drives unfavorable metabolic reactions (those with ∆G°′ > 0).
- When coupled with a reaction that has a positive free-energy change, ATP hydrolysis shifts the overall equilibrium to favor product formation.
Components of Enzyme Factors:
- Many enzyme cofactors and coenzymes, such as coenzyme A, NAD+, and FAD, include adenosine in their structures.
- While they vary in other aspects, they all contain adenosine. Although adenosine itself does not participate directly in enzymatic reactions, its removal from these cofactors typically results in a significant decrease in their activity.
- For example, removing the adenosine nucleotide from acetoacetyl-CoA reduces its reactivity as a substrate for β-ketoacyl-CoA transferase, an enzyme involved in lipid metabolism, by a factor of 10^6.
Chemical Messengers:
- Cells respond to their environment by detecting signals from hormones or other chemical substances in their surroundings. The interaction of these first messengers with cell surface receptors often results in the production of second messengers within the cell, leading to adaptive changes.
- Frequently, the second messenger is a nucleotide. One of the most common second messengers is adenosine 3′, 5′-cyclic monophosphate (cyclic AMP or cAMP), which is generated from ATP in a reaction catalyzed by adenylate cyclase, located on the inner face of the plasma membrane.
- Cyclic AMP plays a regulatory role in nearly all cells outside the plant kingdom. Another important second messenger is guanosine 3′, 5′-cyclic monophosphate (cGMP), which also serves regulatory functions in many cells.