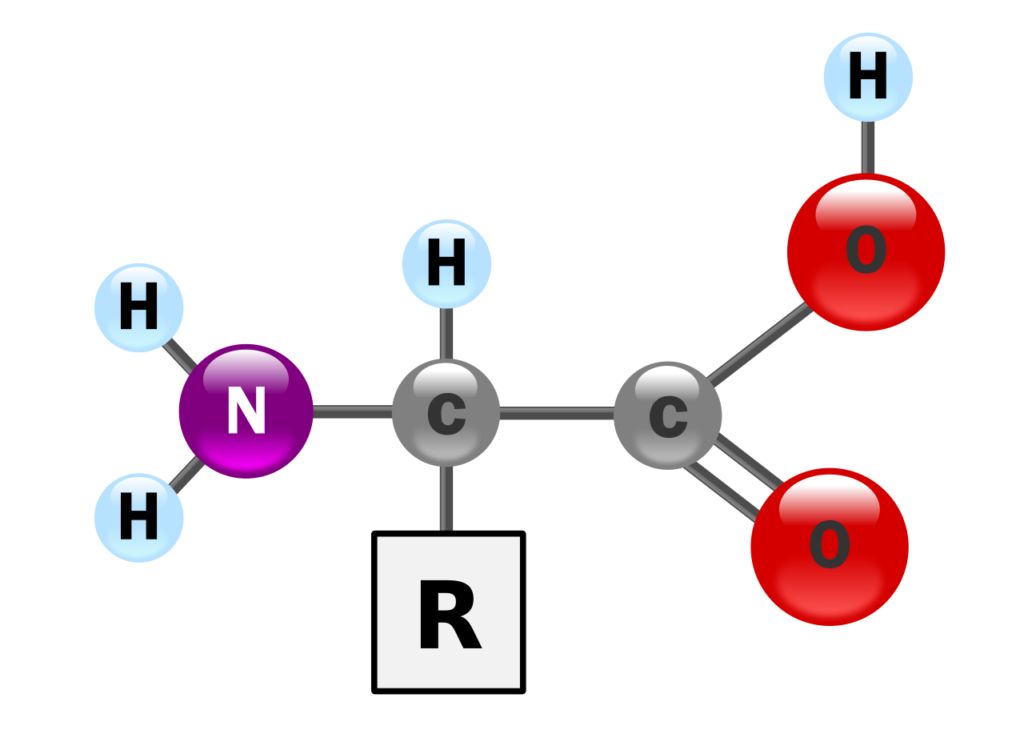
Proteins are macromolecules made up of monomers of amino acids. Each amino acid consists of an amino group, a carboxyl group, and an R group.
The R group, typically carbon-based, gives the amino acid its unique properties. These properties influence molecular interactions, including van der Waals forces between temporary dipoles, ionic interactions between charged groups, and attractions between polar groups.
Proteins are fundamental to life, regulating diverse biological activities such as genetic replication and oxygen transport. They control cellular processes and help determine an organism’s phenotype. It perform their functions through three-dimensional tertiary and quaternary structures formed by interactions with various substrates.
Properties of Protein
- Color and Taste: Proteins are colorless and tasteless. They are homogenous and often have a crystalline structure.
- Molecular Weight: In terms of molecular weight, it is approximately 33 to 55 kilodaltons (kDa), since the average molecular weight of an amino acid residue is roughly 110 daltons.
- Coagulation: Coagulation occurs when proteins such as albumins and globulins undergo heat denaturation, forming insoluble aggregates known as coagulum.
- Solubility: Protein solubility is influenced by the solution’s pH. Increasing the acidity or alkalinity enhances solubility, while at the isoelectric point, proteins are least soluble.
- Optical Activity: Proteins exhibit optical activity due to the asymmetry of polypeptide chains and the α-carbon atoms in amino acids. They are levorotatory, meaning they rotate polarized light to the left.
- Denaturation: Denaturation happens when heat, acids, alkalis, or chemicals like alcohol and urea cause the partial or complete unfolding of the polypeptide chain, disrupting the protein’s native structure.
- Isoelectric pH (pI): The isoelectric pH is the pH at which proteins have an equal number of positive and negative charges. At this pH, proteins are least soluble and do not migrate in an electric field, leading to precipitation.
- Electrochemistry of Proteins: Proteins have redox potential due to charged groups, like the positively charged ammonium groups (―NH3⁺) of lysine and arginine and the negatively charged carboxyl groups (―COO⁻) of aspartic and glutamic acids. These charges influence their movement in an electric field toward respective electrodes (cathode or anode).
- Condensation Reaction: This reaction occurs between the carboxyl group of one amino acid and the amino group of another, forming a peptide bond and releasing water.
Structure of Protein
Proteins are the largest and most diverse group of biological molecules, exhibiting a wide range of structures. Their function is directly linked to their structure. Proteins are formed by linking amino acids through an amide bond known as a “peptide bond.”
Peptide bond forms in a condensation reaction between the alpha amino group of one amino acid and the carboxyl group of another. When two amino acids are joined, the result is called a dipeptide; three form a tripeptide, and several amino acids together form a polypeptide, often referred to simply as a “peptide.”
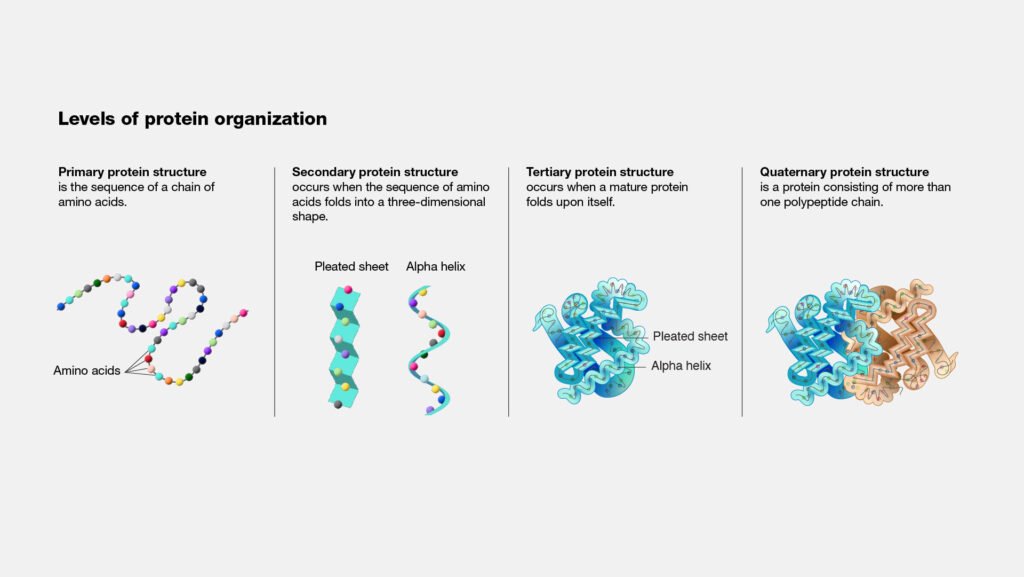
Primary Structure of Protein
The primary structure of a protein denotes the precise sequence of amino acid residues that constitute the protein. It involves only the covalent bonds that connect the residues. A protein is typically defined as having at least 50 residues, while smaller chains are known as peptides. For a small protein, the primary structure would consist of around 50 amino acid residues in sequence.
Secondary Structure
The secondary structure of a protein refers to the folding pattern of the polypeptide backbone, stabilized by hydrogen bonds between N-H and C=O groups. While several types of secondary structures exist, the most common are the alpha helix and beta sheet, which have repeating patterns.
- Alpha Helix: This structure involves a helical arrangement of a single polypeptide chain, resembling a coiled spring. It has specific dimensions with 3.6 residues per turn and a pitch of 0.54 nm. The side chains extend outward, interacting with solvents, creating a shape similar to a bottle brush or round hairbrush. Keratin, found in human hair, is an example of a protein rich in alpha helices.
- Beta Sheet: In contrast, beta sheets consist of polypeptide chains folding back on themselves, lying side by side. These strands are held together by hydrogen bonds, forming a rigid structure. In this arrangement, side chains alternate between projecting upward and downward. Silk fibroin is primarily composed of stacked beta sheets.
Other types of secondary structures include loops, helices, and irregular conformations. A single polypeptide chain may have different regions adopting various secondary structures. Many proteins combine alpha helices, beta sheets, and other folding patterns to create their overall shape.
Tertiary Structure
Tertiary structure refers to the three-dimensional arrangement of a polypeptide chain, which includes the folding of alpha helices, beta sheets, and other loops and folds. This structure is shaped by interactions between the side chains or between side chains and the polypeptide backbone, which may be far apart in the sequence.
The primary driving force for tertiary structure formation is hydrophobic interactions, as polypeptide chains contain both hydrophobic and hydrophilic residues. Other forces involved in stabilizing the tertiary structure include ionic bonds between side chains, hydrogen bonds, and van der Waals forces. These interactions are much weaker than covalent bonds, so multiple weak interactions are needed to stabilize the overall structure.
Quaternary Structure
Some proteins consist of multiple polypeptide chains, and the quaternary structure describes the number and arrangement of these chains, known as subunits. The same forces that stabilize tertiary structure—hydrophobic interactions, ionic bonds, hydrogen bonds, and van der Waals forces—also hold these subunits together to form the complete protein. The subunits can be identical, similar, or completely different.
In certain proteins, subunits are held together by coiled-coils, where intertwined helices are stabilized by hydrophobic surfaces. These surfaces are formed by a repeating pattern of hydrophilic and hydrophobic residues in the alpha helices.
When the helices coil around each other, the hydrophobic sides come together, burying their side chains and creating a stable structure. An example of this is myosin, the motor protein in muscles responsible for contraction.
Classification of Protein
They are divided into different groups primarily based on it shape, solubility, chemical composition and structural complexity.
Classification of Protein based on Solubility and Shape
On the basis of their solubility and shape, it may be divided into two classes: fibrous and globular.
Fibrous Protein
They primarily serve mechanical and structural roles, offering support to both cells and the organism as a whole. They are water-insoluble due to the abundance of hydrophobic amino acids both inside and on their surface. The hydrophobic surface amino acids help them form complex supramolecular structures.
In vertebrates, they provide external protection, support, and shape, giving flexibility or strength due to their structural properties. Some fibrous proteins, such as α-keratins, are only partially digested in the intestine.
- Fibroin: Produced by spiders and insects, such as the silkworm Bombyx mori, fibroin is a key component of silk.
- Collagen: Collagen is a family of proteins rather than a single one. It is the primary protein in connective tissue and makes up 25-30% of all proteins in vertebrates. Collagen is found in tendons, bones, cartilage, and the cornea. Gelatin used in food is a derivative of collagen.
- α-Keratins: These proteins make up nearly the entire dry weight of nails, claws, beaks, hooves, horns, hair, wool, and much of the outer skin layer. Their stiffness and flexibility depend on the number of disulfide bonds, which, along with other forces, stabilize the structure.
- Elastin: Elastin provides elasticity to the skin and blood vessels due to its randomly coiled structure, which sets it apart from the more rigid structures of α-keratins and collagens.
Globular Protein:
Most proteins fall into this category, characterized by a compact, roughly spherical structure that is more complex than fibrous proteins. They are generally water-soluble but can also be embedded in biological membranes.
These proteins serve various structural and mechanical roles, functioning as enzymes, hormones, membrane transporters and receptors, blood transporters for triglycerides, fatty acids, and oxygen, as well as immunoglobulins (antibodies) and storage proteins in grains and legumes.
Examples of globular proteins include myoglobin, hemoglobin, and cytochrome c.
Classification of Protein based on solubility and chemical composition:
On the basis of their chemical composition, they are divided into three different groups i.e, simple, conjugated and derived.
Simple Protein
Simple proteins are also known as homoproteins, consist solely of amino acids and yield only amino acids upon hydrolysis. They can be further categorized based on their solubility in various solvents and their heat coagulability.
- Albumins: These proteins are highly soluble in water, as well as in dilute acids and alkalis, and they coagulate when heated. Albumins can be precipitated from solution by adding high concentrations of salt, a process known as “salting out.” Examples include serum albumin and ovalbumin (found in egg whites).
- Globulins: Globulins are either insoluble or only slightly soluble in water, but their solubility increases significantly with the addition of neutral salts like sodium chloride. These proteins also coagulate when heated. Examples include serum globulin, fibrinogen, myosin from muscle, and the globulins found in pulses.
- Prolamins: These proteins are insoluble in water but dissolve in 70-80% aqueous alcohol. Upon hydrolysis, they release a high amount of proline and amide nitrogen, which is reflected in their name. Examples of prolamins include gliadin from wheat and zein from corn.
- Histones: Histones are small, stable basic proteins that contain a significant amount of the basic amino acid histidine. They are soluble in water but insoluble in ammonium hydroxide, and they do not readily coagulate upon heating. Histones are found in hemoglobin’s globin and in nucleoproteins.
- Protamines: Protamines represent the simplest form of proteins. They are soluble in water and resist coagulation from heat. Their basic nature is due to a high concentration of arginine. Protamines are associated with nucleic acids in the sperm cells of certain fish.
- Albuminoids: These proteins are characterized by their stability and insolubility in water and salt solutions. They are termed albuminoids because they share similarities with albumin and globulins. Albuminoids are highly resistant to proteolytic enzymes and are fibrous in nature, forming a major part of the supporting structures in animals. They are primarily found in exoskeletal structures like hair, horns, and nails.
Conjugated Protein
Also known as heteroproteins, they include a non-protein component in their structure, referred to as prosthetic groups.
- Glycoproteins: These are proteins that have one or more carbohydrate units covalently attached to their polypeptide backbone, typically consisting of no more than 15-20 carbohydrate units. Examples include glycophorin and fibronectin.
- Chromoproteins: These proteins contain colored prosthetic groups. Notable examples include hemoglobin and myoglobin, which bind one and four heme groups, respectively, as well as chlorophylls, which incorporate a porphyrin ring with a magnesium atom at its center, and rhodopsins, which bind retinal.
- Phosphoproteins: These proteins contain phosphoric acid bound to serine and threonine residues. They generally serve structural functions, like tooth dentin, or storage functions, such as the milk caseins (alpha, beta, gamma, and delta) and phosvitin found in egg yolk.
- Nucleoproteins: Composed of simple basic proteins (like protamines or histones) combined with nucleic acids, nucleoproteins are essential components of nuclei and chromatin.
- Mucoproteins: They consist of simple proteins combined with carbohydrates, such as mucopolysaccharides. Soluble mucoproteins are resistant to denaturation by heat and are not easily precipitated by common protein precipitants like trichloroacetic acid or picric acid. The term glycoprotein is specifically used for those that contain a small amount of carbohydrate, typically less than 4% hexosamine.
- Lipoproteins: These are proteins that are conjugated with lipids, including neutral fats, phospholipids, and cholesterol.
- Metalloproteins: These proteins bind metals, such as the globulin transferrin, which can combine with iron, copper, and zinc, accounting for about 3% of total plasma protein. Another example is ceruloplasmin, which contains copper.
Derived Protein
Derived proteins are produced through partial to complete hydrolysis of simple or conjugated proteins, which occurs due to the action of acids, bases, or enzymes. They can be classified into two categories: primary-derived and secondary-derived proteins.
Primary Derived Proteins
These derivatives result from processes that cause minimal changes to the protein molecule and its properties, with little to no cleavage of peptide bonds.
- Proteans: Insoluble products formed by the action of water, dilute acids, and enzymes, typically derived from globulins. They are insoluble in dilute salt solutions, such as myosan from myosin and fibrin from fibrinogen.
- Metaproteins: Created when proteins are treated with acids or bases. They are insoluble in neutral solvents.
- Coagulated Proteins: These are insoluble products resulting from the effects of heat or alcohol on natural proteins, such as cooked meat and cooked albumin.
Secondary Derived Proteins
These proteins are produced through progressive hydrolytic cleavage of the peptide bonds within the protein molecule. They are generally categorized into proteoses, peptones, and peptides based on their average molecular weight.
- Proteoses: Hydrolytic products of proteins that are water-soluble and remain uncoagulated by heat.
- Peptones: These hydrolytic products have a simpler structure than proteoses, are soluble in water, and are also not coagulated by heat.
- Peptides: Composed of a small number of amino acids, these molecules are water-soluble and do not coagulate when heated.
Biological Significance of Protein
Proteins play a vital role in various life-sustaining metabolic processes within organisms. They can be categorized based on their functions into the following groups:
- Structural Proteins: Primarily fibrous in nature, these proteins are insoluble in water and serve as key components in structures such as bones, tendons, cartilage, skin, connective tissue, hair, and horns. Examples include collagen, keratin, and elastin.
- Enzymes: Functioning as biological catalysts, enzymes lower the activation energy required for reactions, thereby accelerating cellular metabolic processes. Most enzymes are globular conjugated proteins. Examples include nitrogenase, DNA polymerase, and lipase.
- Hormones: Protein hormones within cells include substances like glucagon, insulin, and adrenocorticotropic hormone.
- Respiratory Pigments: These are colored, conjugated proteins that contain pigments (chromophores) as their prosthetic groups. Examples include hemoglobin and myoglobin.
- Contractile Proteins: These proteins facilitate muscle contraction using energy derived from ATP molecules. Notable examples are actin and myosin.
- Storage Proteins: Found in seeds, eggs, milk, and pulses, these proteins store metals or amino acids within cells. Examples include casein, gluten, and ferritin.
- Transport Proteins: Responsible for moving molecules or materials to specific locations, these proteins form channels in the plasma membrane and contribute to the formation of blood and lymph in animals. Serum albumin is a common example.
- Defense Proteins: These proteins help protect organisms from foreign microbes or materials. Examples include immunoglobulins (antibodies) and fibrinogen.
- Toxins: Some proteins, such as snake venoms, are toxic in nature.